Good documentation practices
- 2. Good Documentation Practices Q. What is GDP? Good Documentation Practices Q. Why GDP is Important? OK…. Let’s Check The way we understand it..
- 3. Good Documentation Practices What is DP? “Display Picture”
- 4. Good Documentation Practices Why profile pictures are important One is to help other people to identify you. Another is to help you express yourself… and To help others to develop the right impression of you. Similarly…… GDP is important
- 5. Good Documentation Practices Why GDP is Important… Similarly…… GDP is important To Identify who did what To understand what was done To evaluate what need to be To make a correct interpretation for what it meant for Because it is the only evidence to show that what we did, is what we expected to…..
- 6. Good Documentation Practices Why GDP is Important… Similarly…… GDP is important To express yourself, your activity to other who read it like your customer or auditor. To allow them to have correct impression regarding your work and you. To allow them to have assurance on your job
- 7. Good Documentation Practices Why GDP is Important… An essential part of the quality assurance system and should exist for all aspects of GMP (reference: WHO GMP, Volume 2) Good documentation practice is an ‘obvious’ ‘expected’ ‘practice’…! Correct, complete, current, and consistent information effectively meets customer and stakeholder' requirements Helps to reduce observations raised on “inadequate” documentation practices
- 8. Good Documentation Practices What constitutes Good Documentation? Approval, review and updation of documents Changes & current revision status of documents identified Relevant versions of applicable documents available at points of use Documents remain legible and readily identifiable Documents of external origin identified, and their distribution controlled Prevent unintended use of obsolete documents, and archiving
- 9. Good Documentation Practices Observations on poor documentation practices… Document error correction not signed/dated, and didn’t include a reason for the correction Write-overs, multiple line-through and use of "White-out" or other masking device Sample sequence table and audit trail not documented (if its not documented, it didn’t happen) SOP related to production, calibration, storage and maintenance not authorized by the QA head The delegation for the batch release, in case of absence of the QA manager, not recorded / documented Out-of-specification (OOS) procedure not detailed enough; flow chart and /or check-list not available
- 10. Good Documentation Practices Common Documentation Errors…
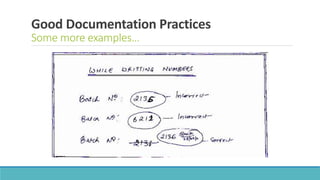
- 11. Good Documentation Practices Some more examples…
- 12. Good Documentation Practices Some more examples…
- 13. Good Documentation Practices Some more examples…
- 14. Good Documentation Practices Some more examples…
- 15. Good Documentation Practices Some more examples…
- 16. Good Documentation Practices What we need to know… Forms of Data, Paper forms, data sheets, and worksheets Notebooks and logbooks Instrument printouts Electronic data obtained with a system such as an electronic data system, laboratory information management system (LIMS), or electronic laboratory notebook (ELN) Categories of documents: Laboratory Records/Procedures Equipment-Related QMS Documents Batch Records Training
- 17. Good Documentation Practices What we need to know… Types of Documents, Specifications: basis for quality evaluation Standard Operating Procedures: gives directions for performing certain operations Batch Manufacturing Records (BMR): gives manufacturing formula and processing instructions Batch Packing Records (BPR): gives details of the packing materials and packing instructions Forms for recording data: used to execute the data of evidence Protocols: describes the details of a comprehensive planned study to investigate the consistent operation of new system/equipment, a new procedure, or the acceptability of a new process before it is implemented Quality System Documents: Change Control, Deviations, Incidents, OOS, OOL, CAPA
- 18. Good Documentation Practices What we need to know… ALCOA & ALCOA+ A- Attributable: Who did what.. L- Legible: One can read what is written C- Contemporaneous: at the time of occurrence O- Original: as obtained and written A- Accurate: must be error free + Complete: No missing + Consistent: Uniform / Sequence + Enduring: Long Lasting + Available: ready to refer We have written procedure to follow: SOP/QAD/GEN/006
- 19. Good Documentation Practices Principles Of Good Documentation… USP <1029> Records should be clear, concise, accurate, and legible. Data entries should be recorded promptly when actions are performed. Backdating and postdating are not allowed. All corrections to the original entries should be initialed and dated (or captured within an electronic audit trail), with an explanation included in cases where the reason for the change is not obvious. Data entries should be traceable to the person who made the entry. Notebooks, data sheets, and worksheets should be traceable. Uncommon abbreviations and acronyms should be defined. If ink may have faded over time (e.g., thermal paper), a copy can be used with verification of its accuracy. An adequate documentation system is needed to ensure data integrity and availability of current and archived records. Controls should be in place to protect the integrity of the records. Records should be retained per regulatory requirements and be readable during the retention period. All pages should be paginated. Attachments (supporting documents) should be paginated with a reference to the parent document.
- 20. Good Documentation Practices What we need to know… Document Should, Be Designed, Prepared, Reviewed, Approved/Authorized, Distributed, And Archived “with care” Comply with the relevant parts of the manufacturing and marketing authorization dossiers. have unambiguous contents; Title, Nature and purpose should be clearly stated Be laid out in an orderly fashion and be easy to check. be regularly reviewed and kept up-to-date. Should prevent inadvertent use of superseded documents. Be provided with sufficient space for entries be made or completed at the time each action is taken Be traceable Any alteration made to the entry on a document should be signed and dated; the alteration should permit the reading of the original information. Where appropriate, the reason for the alteration should be recorded
- 21. Good Documentation Practices What we need to know… Review is important Missing records and out-prints Incomplete entries Illegible corrections Cross References Deviations, if any investigation the impact on the product Valid calibrations and service intervals of test equipment Compliance with specifications, parameter ranges or acceptance criteria including tighter customer specifications
- 22. Good Documentation Practices Some tips on Good Documentation Practices Do’s Records should be completed at time of activity or when any action is taken Superseded documents should be retained for a specific period Records should be retained for at least one year after the expiry date of the finished product Concise, legible, accurate and traceable Picture is worth a thousand words Clear examples Don’ts Enter data when activity has not been completed Sign for work prior to that work being performed Sign for another person’s work with your name Use other than today’s date Change original / non-retrievable data without supporting documentation Document verification of data without individual observation Don’t assume knowledge
- 23. Good Documentation Practices Must-to-have… Errors or mistakes are possible – person recording is human But Practices Makes Perfect Frequently doing something makes one better at doing it
- 24. Good Documentation Practices Must-to-have… All This is achievable through… Positive attitude Positive thoughts Removal of mental blocks Open or willing to change Understand the need of business Destruction of Ego
- 25. Good Documentation Practices Must-to-have… Implementation through… Swear to record the actual information/ data Be honest Strengthen the review Report the deviation Train subordinates on common /identified mistakes or errors
- 26. Good Documentation Practices Must-to-have… Good documentation structure provides an assurance of the quality of product Documentation can be viewed as the foundation of all quality systems Practice puts brains in your muscles
- 27. If you would like to donate us? Scan below and donate us 0.013$ (US dollar) (5Rs Indian rupee) Contact: If you want PPT/PDF files, please contact below. Email: [email protected] Telegram:+919738137533(only for Chat)