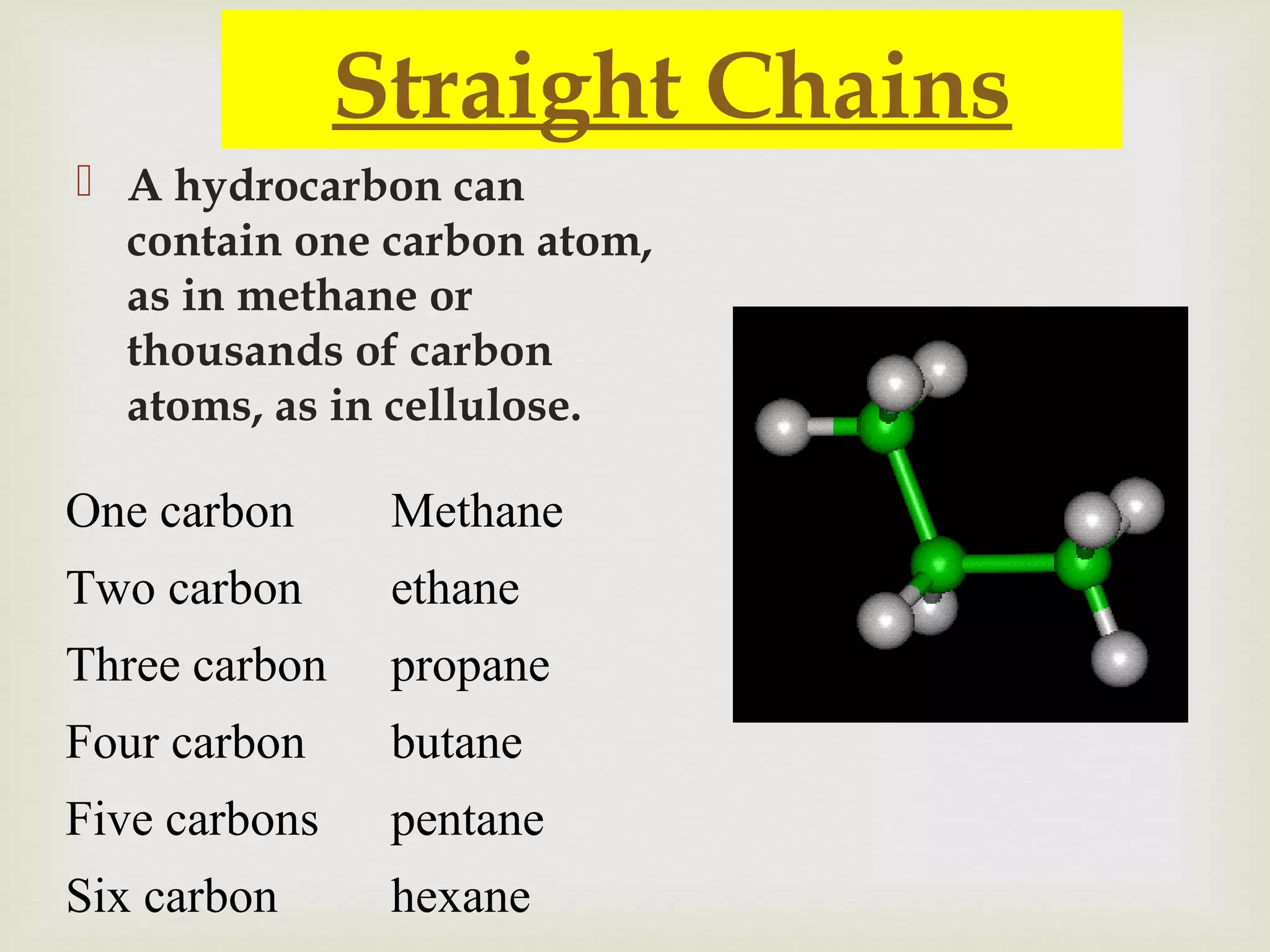
The document provides an overview of organic chemistry, focusing on carbon compounds and their structures, including hydrocarbons and functional groups. It discusses the diversity of organic molecules formed by carbon chains and the properties of saturated and unsaturated hydrocarbons. Additionally, it outlines the significance of polymers and various classes of compounds relevant to biological systems.