Chapter 1 material structure and binary alloy system
- 1. CHAPTER 1 : MATERIAL STRUCTURE AND BINARY ALLOY SYSTEM
- 2. STRUCTURE OF MATERIALS AND EPT
- 3. ATOM Particle Charge Electron Negative (-) Proton Positive (+) Neutron Neutral • Atom is unit of matter; the smallest unit of a chemical element. • Each atom consists of a nucleus, which has a positive charge, and a set of electrons that move
- 4. ELEMENT Is a combination of 2 or more same atoms which form a bond. Atoms in elements all have the same number of protons - i.e. the same atomic number. Example of element; Cl2
- 5. MIXTURE Combination of two or more different substances which are mixed together but are not combined chemically. Example of mixture; salt water, concrete. “Putting Together And Breaking Apart”
- 6. COMPOUND Is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Example of compound; Carbon Dioxide (CO2).
- 7. ATOMIC NUMBER Is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus.
- 8. ATOMIC MASS Is the total number of protons and neutrons in the nucleus of an atom. Atomic Mass = No. of proton + No. of neutron
- 9. ATOMIC ORBITS • Electrons if filled to atomic orbits by using formula 2n2 as below. • The highest no. of electron on the outer shell/orbits is 8. No. of No. of orbit (n) electron First orbit (n=1) 2 Second orbit (n=2) 8 Third orbit (n=3) 18 Fourth orbit (n=4) 32
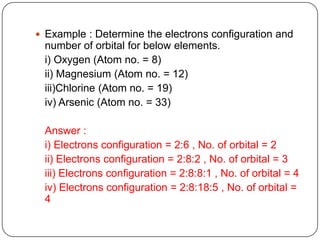
- 10. Example : Determine the electrons configuration and number of orbital for below elements. i) Oxygen (Atom no. = 8) ii) Magnesium (Atom no. = 12) iii)Chlorine (Atom no. = 19) iv) Arsenic (Atom no. = 33) Answer : i) Electrons configuration = 2:6 , No. of orbital = 2 ii) Electrons configuration = 2:8:2 , No. of orbital = 3 iii) Electrons configuration = 2:8:8:1 , No. of orbital = 4 iv) Electrons configuration = 2:8:18:5 , No. of orbital = 4
- 11. Atom’s Number Atom’s Mass 7 14 Element’s Symbol N Nitrogen Element’s Name 2:5 Electrons configuration
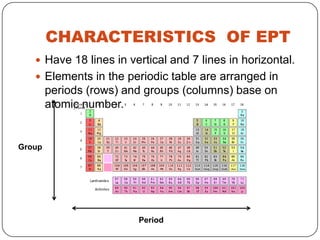
- 13. CHARACTERISTICS OF EPT Have 18 lines in vertical and 7 lines in horizontal. Elements in the periodic table are arranged in periods (rows) and groups (columns) base on atomic number. Group Period
- 14. • Groups - Elements in same group having the same electron configuration in their outer shell. E.g ; element in group 1 has 1 electron in outer shell, element in group 2 has 2 electron in outer shell. - Elements in groups sharing similar chemical properties. • Period - Elements in same period having the same no. of orbit. - Each of the seven periods is filled sequentially by atomic number • When valance electron increase, metal properties of element decrease.
- 15. FUNCTION OF EPT To ease the classification of elements. Able to provide information especially for properties of the elements due to elements are arrange in respective group. Easier to analyze and understand reaction between elements.
- 16. CRYSTALLIZE STRUCTURE The atoms arrange themselves into various orderly configuration, called crystal. Crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. Patterns are located upon the points of a lattice, which is an array of points repeating periodically in three dimensions. The points can be thought of as forming identical tiny boxes, called unit cells, that fill the space of the lattice
- 17. There are 4 types of crystal structure: Types of crystallize structure Body Hexagonal Face centered Simple cube centered Close packed cubic (FCC) cubic (BCC) (HCP)
- 18. SIMPLE CUBE • Is a cube (all sides of the same length and all face perpendicular to each other) with an atom at each corner of the unit cell. • Contains only one atom per unit cell.
- 19. BODY CENTERED CUBIC (BCC) • Is a cube (all sides of the same length and all face perpendicular to each other) with an atom at each corner of the unit cell and an atom in the center of the unit cell. • Contains two atoms per unit cell.
- 20. FACE CENTERED CUBIC (FCC) • Is a cube (all sides of the same length and all face perpendicular to each other) with an atom at each corner of the unit cell and an atom situated in the middle of each face of the unit cell. • Contains four atoms per unit cell.
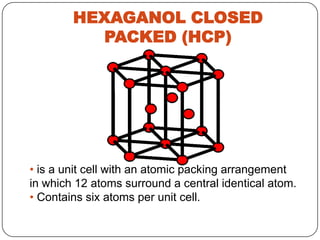
- 21. HEXAGANOL CLOSED PACKED (HCP) • is a unit cell with an atomic packing arrangement in which 12 atoms surround a central identical atom. • Contains six atoms per unit cell.
- 22. COVALENT BOND Bond occur between non-metals. Covalent chemical bonds involve the sharing of a pair of valence electrons by two atoms. Example ; Cl2 !!! Remember : Covalent = sharing atoms
- 24. IONIC BONDING Bonds occur between metals and non-metals. The metal gives its outer electrons to the non- metal - electrons are transferred in this type of bonding. The metal has lost electrons, and is now a positive ion or cation. The non-metal has gained electrons, and is now a negative ion or anion. Both the metal and non-metal now have full outer shells of electrons.
- 25. • Example ; NaCl !!! Remember : Ionic = gain/loose atoms
- 27. METALLIC BONDING The bonding in metal elements is called metallic bonding. In metals, the metal atoms lose their outer electrons to form metal cations. The electrons from all the metal atoms form a "sea" of electrons that can flow around these metal cations. These electrons are "not fixed in one place" or "free to move".
- 28. Metal cations and the electrons are oppositely charged. They will be attracted to each other, and also to other metal cations. These electrostatic forces are called metallic bonds, and these are what hold the particles together in metals.
- 29. SOLIDIFICATION OF METAL AND ALLOYS
- 30. SOLIDIFICATION PHASES First Stage Second Third Stage Fourth Stage Stage Nucleus formation Dendrite grow Dendrite growth & Solidification ends arms meet to form the with the formation of grain boundary grain
- 31. METAL VS ALLOY Metal Alloy Metal is made up of Alloy is a mixture of two only one element. or more metals, or metal and non-metal. Has a relatively high Has a low melting melting point. point.
- 32. SOLID SOLUTION Terms in solid solution > Solute : is the element that is added to the solvent. Eg. Sugar. > Solvent : a liquid substance capable of dissolving other substances. Eg. Water. Solid solution : when solute is added to the solvent, the crystal structure of the solvent remains unchanged and the mixture remains in a single homogeneous phase. 2 types of solid solution : i) Substitution solid solution ii) interstitial solid solution
- 33. COMPARISON ON TYPES OF SOLID SOLUTION Substitution Interstitial Formation : If the Formation : The solute atoms of the solvent atom does not displace (host atom) are a solvent atom (host replaced in the crystal atom), but rather it lattice by atoms of the enters one of the holes solute metal. or interstices between the solvent atoms.
- 34. Atomic size : Solute Atomic size : Solute and solvent atomic atoms must have a radii have the smaller atomic radii difference in atomic less than one radii less than about angstrom compare to 15 percent. solvent atom. Others : Have 2 types of substitution solid solution. Disordered and Ordered.
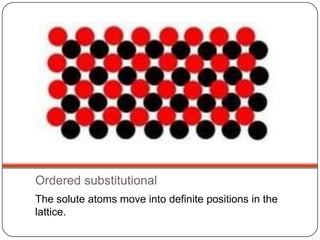
- 35. Ordered substitutional The solute atoms move into definite positions in the lattice.
- 36. Disordered substitutional The solute atoms do not occupy any specific position but are distributed at random in the lattice structure of the solvent.
- 38. Solid Liquid Liquid Solid When certain metal is Kinetic energy for heated constantly, it will atoms increase. start to melt. Atoms arrangement If the heating change from disordered continued, metal will position to certain melt entirely. geometry position. The constant melting Solid materials temperature is melting formed either in point. amorphous or At the melting point crystalline structure. the solid and liquid phase exist in !!! Amorphous : Atoms do not have a long-range crystalline structure. equilibrium Have no grain boundaries and atoms are randomly packed.
- 39. EQUILIBRIUM PHASE DIAGRAM
- 40. Point 100% Cu & 0% Ni - solidification temperature 1084 C Point 80% Cu & 20% Ni - solidification starts at 1190 C. - Complete at 1135 C. Point 80% Ni & 20% Cu - solidification starts at 1410 C. - Complete at 1380 C. Point 100% Ni & 0% Cu - solidification temperature 1445 C
- 41. TERMINOLOGIES IN PHASE DIAGRAM Phase : is a physical distinct and homogenous portion in a material. Equilibrium phase : are graphical representations of what phases are present under equilibrium conditions at various temperature, pressure and composition. Composition : Are percentage of certain materials added purposely or not, to another material. With this it can cause changes in phases, the properties and the shape of the microstructures. Liquidus : The minimum temperature at which all components of a mixture (such as an alloy) can be in a